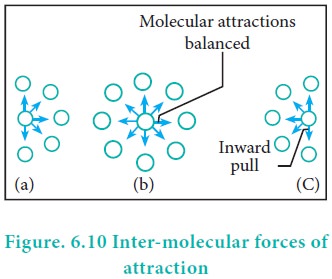
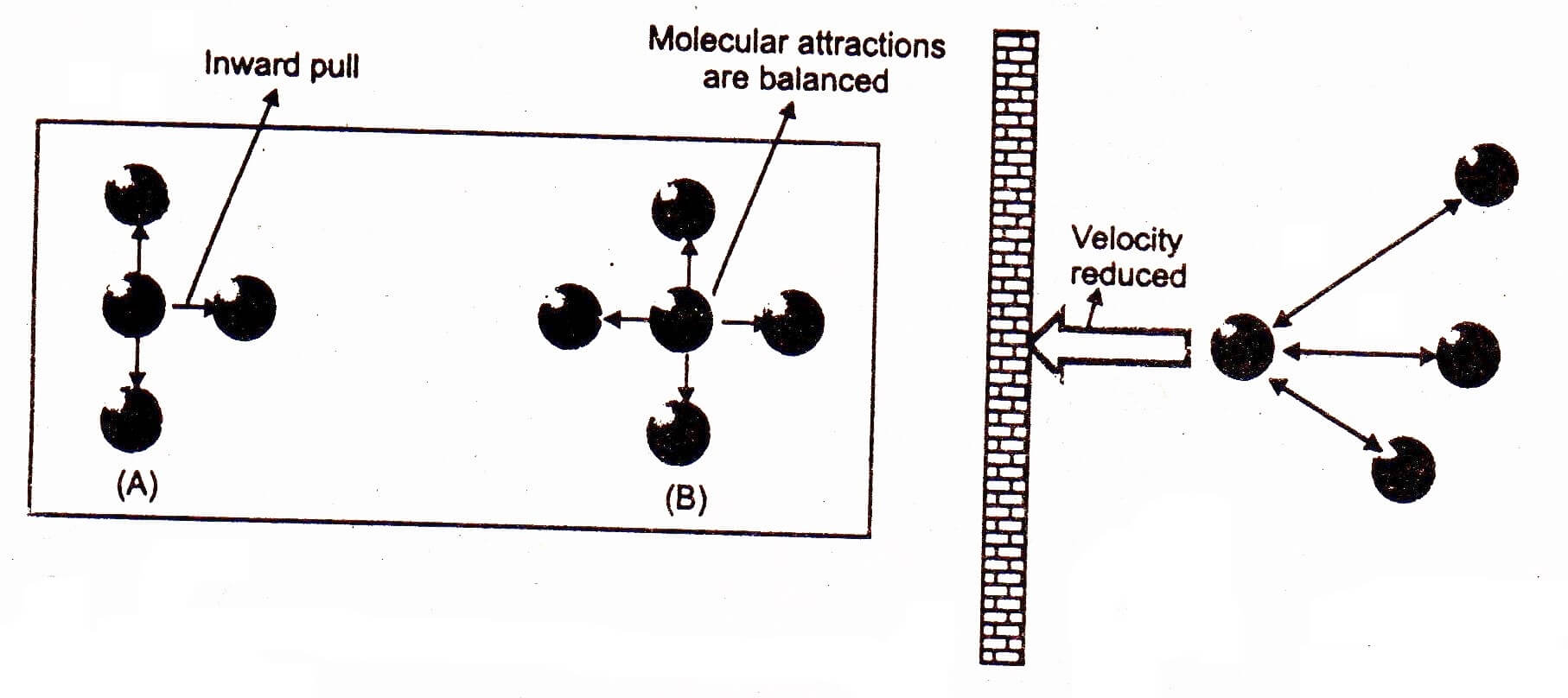
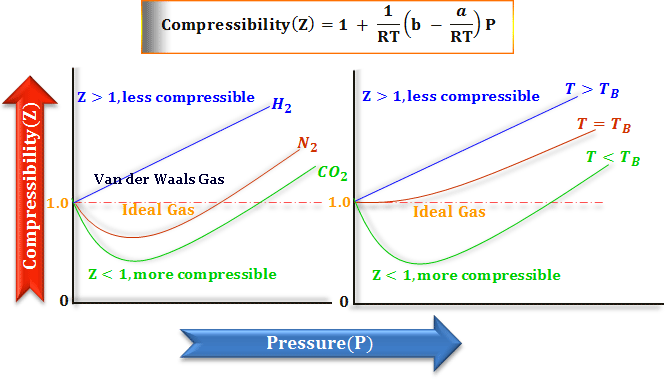
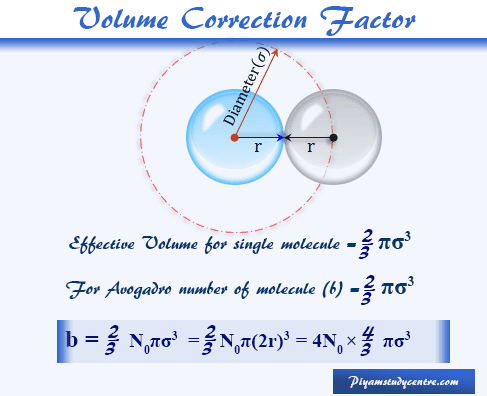
Real Gases: Factors That Cause Deviation from Ideal Behavior 11.6 At high pressure molecules are close together and individual volume becomes significant. - ppt download
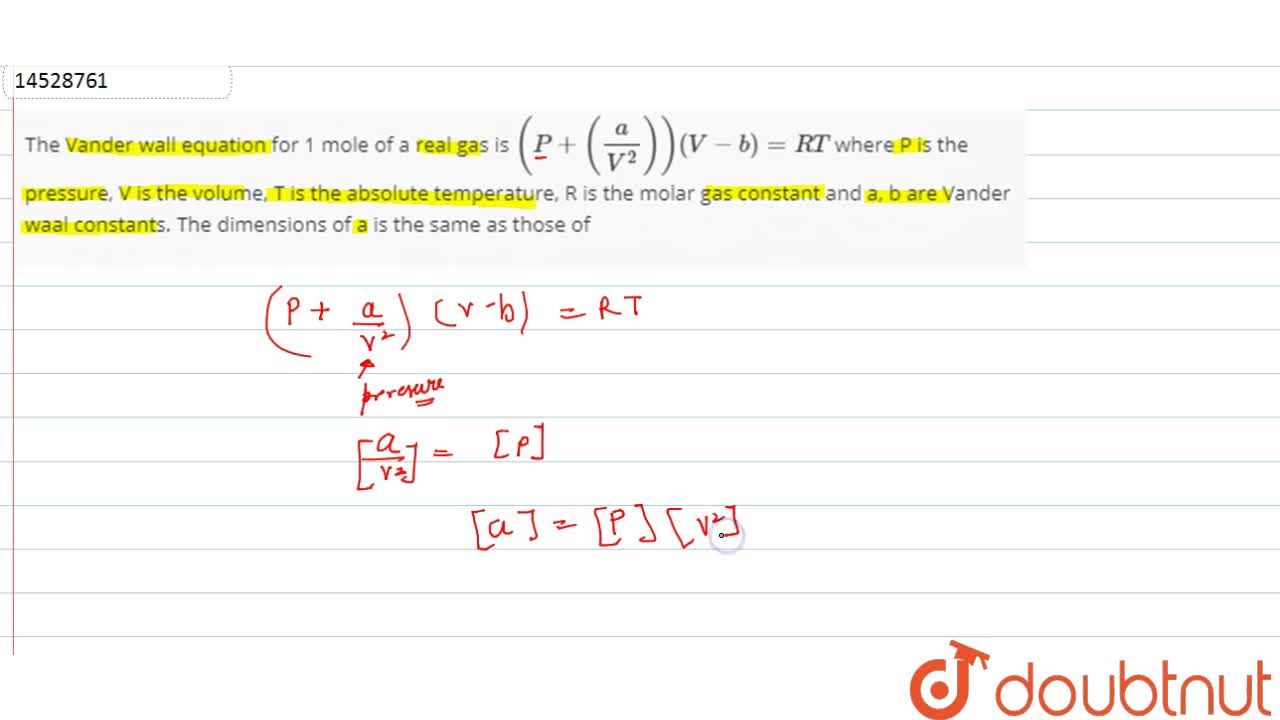
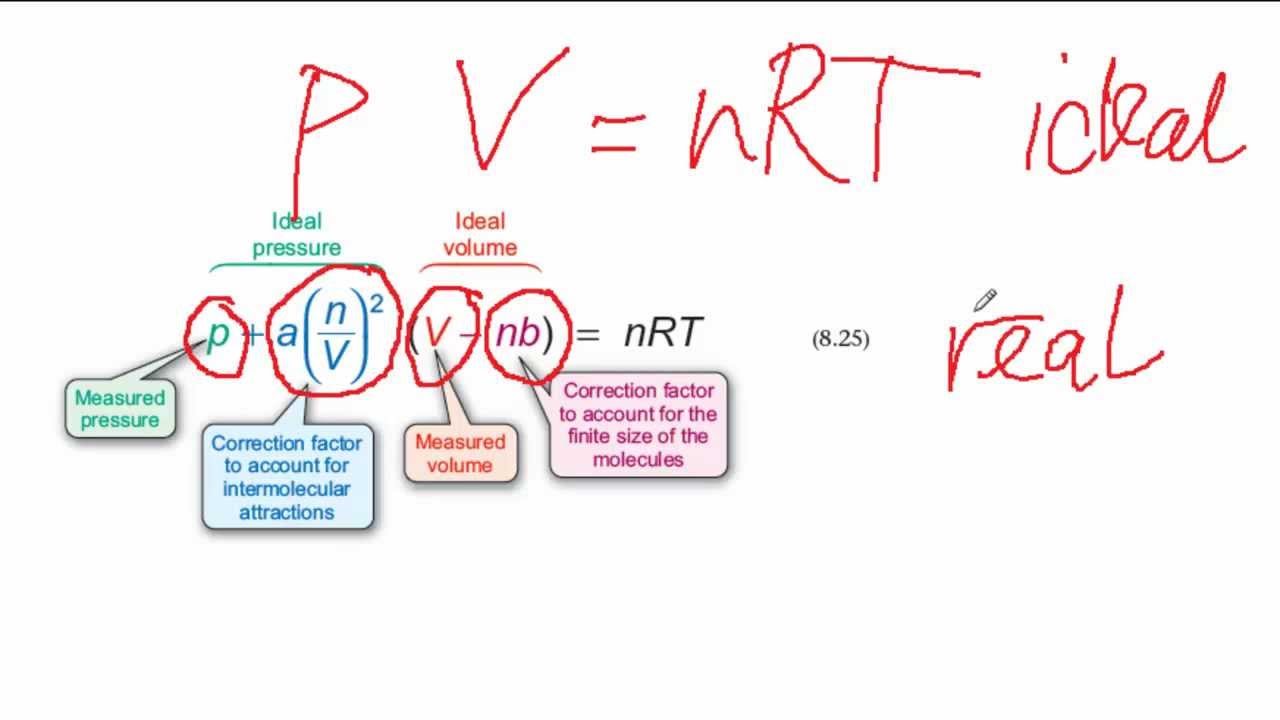
The Vander wall equation for 1 mole of a real gas is (P+ (a/V^2))(V-b) =RT where P is the pressure, V is the volume, T is the absolute temperature, R is the
How real gases are different from ideal gases? Derive van der Waal's equation by pressure and volume modifications. - Sarthaks eConnect | Largest Online Education Community

The Van der Wall equation for 1 mole of a real gas is ( P + a/V^2 )(V - b) = RT where P is the pressure, V is the volume, T